|
|
Post by smartliketruck on Sept 22, 2016 16:46:34 GMT -8
First off, thanks to all the great contributors on this board, so much great stuff going on here. I'll edit this initial post listing with any successes I might have. So far I've been unable to get my locally dug brown clay to polymerize, I suspect that the iron content is too high and the silicon and/or aluminum content is too low. Happily I've gotten Edgar Plastic Kaolin (EPK) to work as a geopolymer cement by treating it with vinegar and then reacting the clay with sodium lye (NaOH). Starts here donkey32.proboards.com/post/22653/threadI don't understand the chemistry of the stages that lead to geopolymerization, right now I'm just sharing getting my hands dirty trying to find a recipe that will work with the materials I have on hand or that I have easy access to. If this was dead simple to understand wouldn't nearly every building site be using geopolymer concrete rather than portland based concrete? Anywho on to my misadventures. Yesterday I picked up some locally dug brownish clay.  I shook a lump up in a glass jar and let it set ~14 hours but the clay stayed in a thin slip/suspension  Here's a lump that didn't stratify or go into suspension in the jar  I mixed 10kg of the clay with 2.5 liters of 10% acetic acid cleaning vinegar resulting in a batter a little thicker than a cake batter  I heated and further mixed the batter on the propane grill  I brought the temperature up till I didn't feel like watching it anymore it got up to 55 C, I then wrapped the pot in wool blankets to hold the heat in longer  I'd like to mix this acid treated clay with untreated clay as aggregate in a solution that has about the same consistency so it is easy to mix with my drill along with with sodium hydroxide or sodium silicate. I have 6kg of sodium hydroxide flakes and 6kg of silica gel crystals available. Does anyone have suggestions of proportions of moist clay aggregate to sodium hydroxide or sodium silicate to try with this acid treated clay? |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Sept 22, 2016 17:10:04 GMT -8
It is not possible to make suggestios to an unknown wet clay. There are huge differences in water demand.
For one clay 40% water per weight are enough to make a
thick paste another may require 100%.
Dry a bit clay so that you can check how much water is required to get a slurry like the one you have now.
Then you can make a raw calculation of the solid content.
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Sept 23, 2016 4:31:33 GMT -8
Let's assume your clay soil contains 25% water,
then you have mixed 7.5 kg clay with 250g acetic acid,
which gives 3.33% acetic acid.
7.5 kg clay + 5kg water gives a 60% solution.
1kg of your slurry would contain 600g clay.
Let's assume your clay soil contains 35% water,
then you have mixed 6.5 kg clay with 250g acetic acid,
which gives 3.85% acetic acid.
6.5 kg clay + 6kg water gives a 52% solution.
1kg of your slurry would contain 520g clay.
Let's assume your clay soil contains 40% water,
then you have mixed 6 kg clay with 250g acetic acid,
which gives 4.2% acetic acid.
6 kg clay + 6.5kg water gives a 48% solution.
1kg of your slurry would contain 480g clay.
Judging from the picture your slurry may contain more than 50% water.
In any case there is already to much water for additional liquid waterglass.
You could try a variant of the LTGS binder by dry weight.
Assume your slurry contains 50% water.
30% clay, 30% silica gel and 40% lye with as little water as possible.
You will need to heat up the mixture to dissove the silica gel.
Try to mix a small part of your slurry, by dry weight, with 30% of the binder above.
Maybe rather 40% binder.
Alternatively you could use your activated slurry exclusively for making a LTGS binder
to be mixed with untreated moist clay soil to a LTGS ram mixture.
As your slurry contains rougly 50% water you could mix it with 50% lye flakes
to get a rougly 1/1 ratio by dry weight.
I do not know how long such a binder could be stored at ambient temperatures if sealed.
Assumed your moist clay soil contains 35% water and the 65% clay are 90% of the final
ram mixture you can easily calculate how much of the wet binder would be required
to give 10% by dry weight of the dry solids.
With wet clay you cannot be certain about the true ratios.
For precise formulas you need to dry your clay soil first.
|
|
|
|
Post by smartliketruck on Sept 23, 2016 14:42:11 GMT -8
A 100g piece of my clay in it's "natural" wet state has a volume of 50ml, dried out it weighs 78g.
The only liquid added to make the slurry was the bottle of vinegar and I must add I didn't see any bubbling when the vinegar was added
Therefore current slurry is
Natural Clay dry weight 7.8 kg
Water included in clay 2.2 kg
Water from vinegar 2.25 kg
Acetic acid 0.25 kg
I'll ponder this and come back soon.
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Sept 23, 2016 16:20:16 GMT -8
Your clay has a relatively low water demand,
which is good.
Why did you expect bubbling ?
Without salts of carbonic acid bubbling is not to be expected.
|
|
|
|
Post by smartliketruck on Sept 23, 2016 20:47:02 GMT -8
There's still a fair bit of acetic acid in the slurry according to my Mark I nostrils that were recently random calibrated.so I put the pot on the burner for a few hours and it's now tucked in safe and warm for the night.
The pot of slurry was thicker viscosity wise and seemed to progressively get thicker as I was stirring the pot. Getting it on the weigh scale will tell me whether the clay is swelling from the acid and heat or simply getting thicker by dehydration.
Tomorrow I'll mix a puck or two with lye crystals, hopefully I'll notice a property change as I cross over from sodium hydroxide + acetic acid = sodium acetate + water to loading the mixture with sodium hydroxide.
Speaking of sodium acetate, both the anhydrous and trihydrate could be useful further down the line in the heat storage department.
After examining the lump I dried out to ascertain water content of the straight outta the ground clay, I feel confident even low end strength gain will be sufficient for my needs.
I should state my initial goal of this project is to create a riser tube and if I have to resort to agricultural perlite or roxul compressed batting as an aggregate then so be it.
Karl: I guess the reason I was expecting bubbles is because most clay I see is in the form of bentonite based drilling mud with a high ph from sodium hydroxide, calcium carbonate and other additives.
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Sept 24, 2016 4:07:27 GMT -8
The pot of slurry was thicker viscosity wise and seemed to progressively get thicker as I was stirring the pot. Getting it on the weigh scale will tell me whether the clay is swelling from the acid and heat or simply getting thicker by dehydration. Both plays a role, increased surface by the acid and dehydration. A ram mixture with 10% LTGS binder is the cheapest way, as it requires only 5% lye and no waterglass to get hard like firebricks. Anhydrous sodium acetate decomposes at 324 °C and the trihydrate at 58 °C. Geopolymers have a low thermal conductivity by nature. Even a LTGS ram mixture with very low water content comes close to an aerogel and can meet the required 50% porosity easily with a bit more water. Nothing can beat an aerogel except vacuum or another aerogel. |
|
|
|
Post by smartliketruck on Sept 26, 2016 0:18:41 GMT -8
The slurry wound up much a much thicker sticky putty weighing in at 10.65kg so nearly two liters of water boiled out. I took 1kg of the mixture and folded 50g of lye beads in First change noted was the mixture quickly lost it's vinegar smell and took on an odd scent that reminds me of cat urine. The mixture very quickly stiffened up to the point where it could only be worked by slapping and pounding. I worked the mixture until I could no longer see any remains of the 1.5mm beads. I formed a tile by hand and put it on the propane grill to dry, after I figured the majority of the water was driven out I put the burner on maximum heat. The thermometer scale on the grill lid only goes to 350C but judging by the needle position it did achieve over 450C The cat urine smell persisted and increased during the firing, the smell dropped off considerably near the end of the firing. The smell is now barely detectable on the tile. The fired tile weighed in at 746g, i'd estimate 10g to 20g of finished material would have been lost to mixing utensils, work table surface and a few stones picked out.  The tile was spot heated on one side with a propane torch for 5 minutes, the opposite side reached 180C, after 20 minutes the hottest spot on either side was 80C.  The tile crumbled easily in halves and smaller but this was not surprising due to cracking from the heat accelerated drying and voids introduced by folding a material that was too stiff. The pieces between the voids and cracks were hard and solid enough to be used as soft bricks, I wouldn't quite say it had a ring to it but not far off.  I think this mixture brought back to a thin paste would make an effective binder for perlite or roxul aggregates. If this mixture was dried very slowly to avoid cracking it would make an effective brick on its own. My clay didn't seem to use up enough of the acetic acid in the vinegar, as lye was added I think a significant amount of lye was used up in the reaction with the left over acetic acid forming sodium acetate. I suspect the sudden stiffening of the mixture was due to a bloom of sodium acetate trihydrate crystals as a turn to just anhydrous sodium acetate would also have free water Next batch of activated clay I make up I will either try regular 5% household vinegar or use more clay with the 10% vinegar as my clay does not seem to need the current ~3% by dry weight I'll see if I can mix a little acetic acid and sodium hydroxide together tomorrow to see if the odd smell is sodium acetate. A tile made with 100g of lye added to 1kg of the current acid activated clay is forthcoming. Clear as mud? |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Sept 26, 2016 3:29:56 GMT -8
I would recommend to read my threads again and then make a new attempt.
|
|
|
|
Post by smartliketruck on Sept 26, 2016 12:47:07 GMT -8
Karl: I've poured over your threads multiple times and have seen lye ratios from 5% to 60% and it seems to me that the ratio is determined by how much silica surface is available for the lye to react with or bond to. Thus when dealing with local clays of unknown mineral contents and surface areas, adjustments to the recipes are necessary.
Is there a key point that you think I'm not understanding?
At this point after two trials I've determined that I need to reduce the amount of acetic acid to avoid wasting lye, plus the smell generated is unpleasant. Because of the smell I can not fire the pieces in my kitchen oven on self cleaning mode, electricity here costs less per thermal unit than bottles of propane.
My goals for now will be along the lines of good enough is good enough with the least amount of dollars, material and labour to get there.
I'm thinking of the 80%/20% principle or Praedels Theorem.
The second trial with 100g of lye resulted in a better product than the first but the preexisting gravel in the clay is proving to be the next hindrance to be overcome.
I'll post the process and results of the second trial tonight.
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Sept 26, 2016 13:32:12 GMT -8
Read my thread about LTGS binder donkey32.proboards.com/thread/2070/karls-brick-cheap-geopolymer-refractoryYou need to acid activate only a very small amount of the clay for a ram mixture. If you want to use large amounts of aggregate you need not only lye but also waterglass. Again, only a small part of the mixture requires acid. It does not look like you have realy read my threads carecully and very obviously you did not understand anything. Read it again. This is my last answer for you. Do whatever you want, I will not help you. |
|
|
|
Post by smartliketruck on Sept 26, 2016 22:56:42 GMT -8
Well, my bumbling search with two hands and a flashlight continues. I mixed a small amount of lye and regular vinegar and heated to a boil, the funky cat urine smell was there but barely perceptible, not sure why it smells so strong in the clay mixture. A second activated sample of 1kg wet weight was mixed with a solution of 100g of lye and a little more water than it took to completely dissolve the beads. The lye solution was folded into the clay gradually, as it was incorporated in a quick stiffening and smell were again evident. The mixture loosened considerably as the rest of the solution was added, in the end the mixture was a little wetter and stickier than it started. Because of the higher concentration of lye I didn't dare hand knead this mixture even with my nitrile gloved hand, this caused me to miss plucking out a significant amount of gravel from this sample. A tile was formed approximately 2.5cm in depth and dried for 12 hours at ~100C. Tile was then fired at ~500C for four hours.  This tile still had significant fissures from curing and firing, the mixture should have been better checked for gravel as there was a lot 1cm and up in this sample compared to the first. The tile crumbled with little hand force in areas with fissures and gravel, smaller pieces between were even stronger than the first sample.  The tile broke in half before I could do the heat penetration test so it's a half apple to apple comparison with the first sample. 5 minutes of propane torch showed 110C on the opposite side.  A especially bright note is I saved, dried and fired a scraping from the mixing board, I can grab it firmly between one finger and thumb on each hand but cannot break it in half. Since this scraping had a higher surface area to volume ratio, it dried without fissures and because it was a thin layer scraped off the board it had very little gravel in it. From round 1 and 2 my takeaways are 1. slower drying or more surface area are required to prevent cracking, I was trying to ride the edge of boiling while curing this sample before firing and failed to the high side 2. the gravel must be screened out 3. the second sample was significantly stronger than the first between the faults, a higher percentage of lye sample is upcoming. 4. A piece of roxul compressed batting simply sitting atop of the propane grill lid caused a significant shortening of heat up time and an increase of maximum temperature 5. I'm living up to my name in the eyes of some onlookers here  |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Sept 27, 2016 4:43:12 GMT -8
You behave like the opposite of smart.
You have any right to make your own mistakes,
just do not blame later someone or something else.
Try potassium lye, smells a lot better.
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Sept 29, 2016 6:45:33 GMT -8
Little hint for the others:
smartliketruck in his unfathomable wisdom and blessed with a fantastic nose claims leftover acetic acid,
but unfortunately:
Acetic odor is no proof of leftover acetic acid.
Many acetates smell like vinegar.
|
|
|
|
Post by smartliketruck on Sept 30, 2016 13:29:18 GMT -8
Water drawn off of top of slurry that sat for two days on the left, household 5% acetic acid vinegar on the right  Well water on left, well water with 12 beads of lye on right 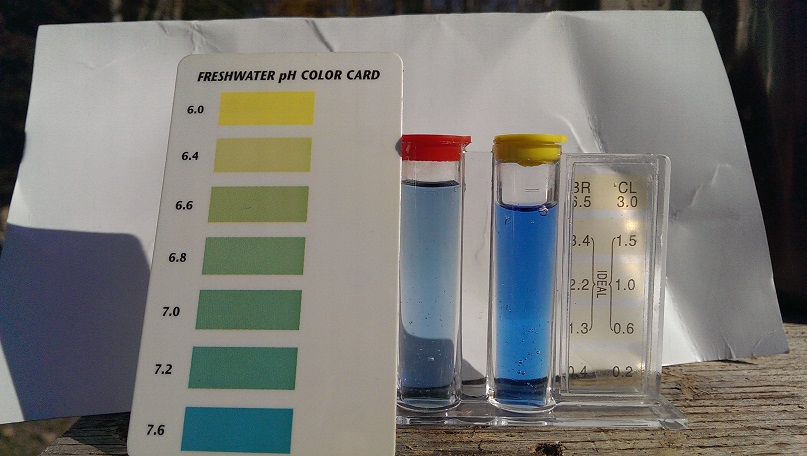 |
|





















