terry
Junior Member
 
Posts: 128 
|
Post by terry on May 30, 2016 0:46:37 GMT -8
Mixing in the batch box
The air which enters the combustion chamber is streaming front to rear, through the port into the riser and from there through a bend 90 degrees up, out of the riser. Namely during the passage through the port the gas velocity is higher as compared to that in the firebox or riser. This phenomenon is known as "the venturi effect", a law of physics first described by Daniel Bernoulli in the 18th century. Due to the fact that the secondary inlet ends directly over the top of the port's entrance, pressure is locally so much lower that air is sucked in automagically provided the velocity of the gases through the port is high enough.
There's another phenomenon which is less known but nevertheless does play an important role in this: the turbulence behind the orifice of the port. This can be considerable, caused by the molecules losing speed abruptly behind the orifice and as a result are coming into collision with the molecules which are racing through the port. It looks like a massive pile-up which is commencing continuously as long as the velocity in the port is higher than in the riser. This will form a double vortex in a more or less horizontal plane which as a double corkscrew spirals up in the riser.
The net effect is violent turbulence and prolonged stay in the riser due to the longer trajectory. Since the riser is surrounded by insulation chances are significant that the temperature in the riser will stay high during mixing. Whereby the reaction with oxygen will take place relatively easy. To demonstrate what this phenomenon looks like see this video.
Hmm, yes, there is quite a bit here I have included in the preceding section grrrrrr. It's a bit of a bugger to break up the 'flow' of the preceding section, well I think it flows. And I agree, to simply repeat it in this case would be as subtle as a brick in the face. Apart from the video, there is not a lot of new data here. double grrr.
I am a bit stumped to be honest. For example, in the preceding section (turbulence) your original simply mentions 'it occurs' really. "In the batch box design a different system to induce turbulence is employed. To be precise, due to the shape of the core and the positioning of the air intakes." It then talks about the 'parts' and sizes of the parts and where the secondary air is placed, and ends with 'Due to the fact that turbulence isn't induced primarily by incoming air the total cross section area of the combined air intakes is smaller than would be expected in a normal box stove. Especially when the speed of fuel consumption is considered.'
The 'fact' of turbulence, yet no explanation of how or why there IS turbulence. I guess that was the hole I was trying to fill. It seems a bit redundant to now talk about 'mixing' (caused by the principles just covered) as somehow different to turbulence (caused by the principles just covered), turbulence (almost by definition) is kinda mixing anyway.
But I do agree with you, we are certainly at a 'clumsy repetition' of data.
Is it actually needed that there is a separate section for mixing and one for turbulence?? (oh, while I remember, it seems the video of a fine demonstration of the flammabilty of smoke has gone??)
Whilst you ponder that question, I'll do another section while I have a bit of time.
This thing is generating also a typical noise, which is louder when the core is placed inside a hollow metal vessel. See this video to experience what it sounds like. In the frequently used brick housing the sound is still audible, like a sort of gargling and rumbling but not disturbingly so.
These heaters generate a typical noise, a low rumbling (but strangely comforting) sound. In fact, it is this characteristic sound found in all of these variants of heaters that gave the name 'Rocket Stoves'. The short video that follows gives an indication of this characteristic sound, this particular example is in a metal housing so has more of a 'ring' to it, builds that employ masonry or brick housings the timbre changes to a less ringy, lower rumbling sound that is not offensive at all.
Lots of heat
By applying sufficient insulation around the firebox but especially around the riser the optimum temperature of the core will be reached earlier. Not only that, also the flammable properties of the woodgas and air mix is increased greatly. In turn, this has a positive effect on efficiency, more gases are burned and complete combustion will start much earlier in the burn cycle.
Both the firebox and heat riser are insulated heavily, with particular emphasis on insulation of the heat riser (where the temperatures can be the greatest). This enables the whole to more quickly reach optimum operating temperature and enhances the combustion of the woodgas/oxygen mixture which is of course the basis of heater efficiency.
Restrictions
The whole of this core is fairly critical as far as shape and sizes are concerned. This is quite logical, the shape and ratios are largely resposible for what's going on in there. A proper chimney is required because the clean combustion is only achieved at a sufficiently high gas velocity. The air intakes are fairly limited so a small decrease or increase of the air supply could have markedly effects. When the chimney temperature and thereby the draft increases it is advised to decrease the main air inlet opening.
The original design is always running flat out so a steel surrounding which is transferring the heat to the air around it should be quite large. Use of a casing built out of brick or other mass materials to store the heat is obvious but this will be large as well due to the requirement of lots of weight.
But... when the thing goes it will do its job remarkably well. When fuel nature and humidity in one and the same appliance are equal, regardless of the load, there will be a small tolerance in the expired time from lighting to glowing phase. A full load compared to half a load will be done in approximately the same time frame so the full load will deliver a huge amount of energy because of this phenomenon.
Hmm, restrictions. Not sure if this is the correct word to use (and I see above that you used limitations). Am trying to think of a more appropriate word...'caveats'?? 'Warnings'?? 'Cautions'?? You are probably thinking 'Hey, I don't know, you are the one fixing the english, not me' and want me to make the decision. Choose any of the above if you think correct, but what if we went 'Important point'?? That seems to work in the short explanatory paragraphs, and I think it will work here. Your choice.
It is important to realise that the shapes and dimensions of the combustion unit are quite critical, variations from these are effectively untested (you variation may have hit upon a winner, but without testing no-one will ever know). The 'tightness' of these dimensions and ratios is quite logical, they are responsible for what's going on in there.
To achieve the goals of this heater (smoke free highly efficient combustion that can be followed and built by others) it is important that the developed and tested dimensions are followed quite closely.
A proper chimney is required, a chimney is the 'engine' of any wood combustion heater, and is the motive force that creates sufficient draft for clean combustion. As discussed above, the air inlets are smaller than 'expected' and as such are perhaps more easily affected by variations from the design given here. [note, I don't recall yet any discussion of air intake ratios???] When the flue temperatures rises (and hence the draft) the air inlet can be decreased, or larger pieces of wood used. Larger more 'chunky' pieces of wood have less surface area than a similar weight of 'finely divided' wood.
These heaters burn the fuel load without restrictions in the air supply or any other measure used to 'slow the burn down for longevity'. It should be clear by now that for maximum efficiency and cleanliness of the burn those types of measures only harm that goal. And so to harness or profitably utilise the heat created we need a large radiating surface or a sufficiently large mass to absorb and slowly release the stored heat. These different approaches will be covered later.
A curious phenomenon of these heaters is the time taken to burn a load. It turns out (rather counter-intuitively) that full load of wood combusts in about the same time as a half load of wood (or other ratio), from lighting to glowing ember phase. So it can be seen that a full load of wood delivers a surprisingly large amount of energy in a given time. Hence we need ways to harvest this heat which will be discussed in the following pages.
That's enough for now, you have this to digest and the earlier questions.
as always, keep reject or modify as seen fit.
|
|
|
|
Post by peterberg on May 30, 2016 2:16:59 GMT -8
So in the story the first bed is too long, the second bed is too short, the third bed is just right. The first chair is too hard, the second chair is too soft, the third chair is just right. The first porridge is too hot, the second porridge is too cold, the third porridge is just right. Pretty sure you've got the drift now  OK, I get it. In my native language this tale is also known, but we don't refer to it in the way you do. I think we'll leave it where it is and link it to the Wikipedia article to facilitate the non-native speakers. Is it actually needed that there is a separate section for mixing and one for turbulence?? (oh, while I remember, it seems the video of a fine demonstration of the flammabilty of smoke has gone??) No, it isn't. Maybe you could mix both parts together? Pun intended. My browser shows the video alright, it's at the end of the "Wood fire" section. I f****d up your message, sorry. I'd hit the edit button instead of the quote button, won't happen again if I can help it. |
|
terry
Junior Member
 
Posts: 128 
|
Post by terry on May 30, 2016 3:41:33 GMT -8
So in the story the first bed is too long, the second bed is too short, the third bed is just right. The first chair is too hard, the second chair is too soft, the third chair is just right. The first porridge is too hot, the second porridge is too cold, the third porridge is just right. Pretty sure you've got the drift now  OK, I get it. In my native language this tale is also known, but we don't refer to it in the way you do. I think we'll leave it where it is and link it to the Wikipedia article to facilitate the non-native speakers. Is it actually needed that there is a separate section for mixing and one for turbulence?? (oh, while I remember, it seems the video of a fine demonstration of the flammabilty of smoke has gone??) No, it isn't. Maybe you could mix both parts together? Pun intended. My browser shows the video alright, it's at the end of the "Wood fire" section. I f****d up your message, sorry. I'd hit the edit button instead of the quote button, won't happen again if I can help it. If you link to wiki, can you link to that exact section? otherwise perhaps that subtle point is lost. I'll have another look tomorrow, but for now why not just copy and paste that section we are talking about, add the bit about the video from the following section, it might all happily work just as it is bar a few tweaks. If I can see it on your pgae it is easier to see where mods are needed than from copy and paste on this site. If any of that made sense. I just had a look, I don't see that smoke re burn, am pretty sure it was there a few days ago. will check it tomorrow. |
|
|
|
Post by peterberg on May 30, 2016 5:59:02 GMT -8
I'll have another look tomorrow, but for now why not just copy and paste that section we are talking about, add the bit about the video from the following section, it might all happily work just as it is bar a few tweaks. If I can see it on your pgae it is easier to see where mods are needed than from copy and paste on this site. If any of that made sense. I just had a look, I don't see that smoke re burn, am pretty sure it was there a few days ago. Makes sense, I'll do the copy and paste so it will be easier for you. Just checked, the video is in plain sight. Maybe you expect it to be somewhere else? |
|
terry
Junior Member
 
Posts: 128 
|
Post by terry on May 30, 2016 13:37:33 GMT -8
yep, the video is back. well that was rather odd!
It all looks easier to see what is what now it is there, helps a lot actually. I think we can get away with only a few changes, see how it works for you.
How about this is changed Turbulence in the batch box to Mixing and turbulence in the batch box. In this way it directly mirrors the previous section 'mixing and turbulence' and leads directly and naturally from the last line 'The heaters described here are designed to maintain the hottest burn temperatures possible -far greater than metal heaters can withstand- and ensure proper mixing of the fuel gas and oxygen by methods described in the following sections.' (maybe the plural is not needed as only one section explains it) This would mean an appropriate modification to your bullet point introduction.
If that works for you also, then a few more changes and we will be sweet. This 'Mixing in the batch box' is no longer needed as a separate section and any points not stated can be incorporated, especially the video which sums it all up nicely.
'The thorough and complete mixing of the wood gas and oxygen occurs as the mixture passes through the narrow port and into the heat riser behind. As the gas flow passes through it speeds up, when it suddenly hits the *larger space behind* it suddenly slows down again (remember, we have the same amount of gas at each point flowing through the system, so it speeds up and slows down as required in order to maintain the same flow rate). This sudden slowing down creates a highly turbulent environment in an extremely hot space.'
I liked/preferred your explanation, so will incorporate it.
'There's another phenomenon which is less known but nevertheless does play an important role in this: the turbulence behind the orifice of the port. This can be considerable, caused by the molecules losing speed abruptly behind the orifice and as a result are coming into collision with the molecules which are racing through the port. It looks like a massive pile-up which is commencing continuously as long as the velocity in the port is higher than in the riser. This will form a double vortex in a more or less horizontal plane which as a double corkscrew spirals up in the riser.
The net effect is violent turbulence and prolonged stay in the riser due to the longer trajectory. Since the riser is surrounded by insulation chances are significant that the temperature in the riser will stay high during mixing. Whereby the reaction with oxygen will take place relatively easy. To demonstrate what this phenomenon looks like see this video.'
The thorough and complete mixing of the wood gas and oxygen occurs as the mixture passes through the narrow port and into the heat riser behind. As the gas flow speeds up through the restriction of the port and then slows down abruptly when it reaches the *larger* space behind the port, a massive pile up in the gas flow occurs as the still fast moving molecules passing through the port slam into the suddenly slowed molecules in front of them. This creates considerable turbulence and is continuous as long as the velocity of the gases are higher in the port than in the riser, the large majority of the burn time. These conditions cause the flammable gases to mix into a swirling double vortex in first a horizontal plane and then an ascending double corkscrew as it rises within the heat riser as it exits the system.
The ascending double corkscrew forces the gases to take a much longer path (and hence take longer time) than if they were to go straight up. That this longer travel occurs within a well insulated, extremely hot environment allows the mixed fuel and oxygen to readily combust.
The speeding up of gases as they pass through a restriction is known as "the venturi effect", a law of physics first described by Daniel Bernoulli in the 18th century.
The highly chaotic conditions created by this arrangement can be seen in this short video filmed looking down the heat riser and directly at the exit of the port, where the massive pile up occurs and the double vortex/ascending corkscrew is formed.
This might need a re-arrangement of the videos as the rest of the earlier section follows on. It looks like this sentence can go 'We have just created the perfect set of conditions for complete combustion. Thorough mixing of fuel and oxygen in the presence of extraordinary amounts of heat.'
I THINK that should all flow now, up to 'results', but it is honestly a bit hard to tell! It is very much easier to see once the changes are made. I am sure you can iron out any wrinkles that might appear, so lets give that a shot and then carry on if all is well.
|
|
terry
Junior Member
 
Posts: 128 
|
Post by terry on May 30, 2016 19:30:59 GMT -8
let's push on to the end!
'Results
In the course of four years I've executed many hundreds of test runs on this core and in the majority of circumstances the results are commendable. The Testo 330-2, the gas analyzer which has been used to measure a test is able to connect to a computer and the software generates its own line diagrams and spreadsheets.'
In the last four years I've executed hundreds of runs on the core presented here, and am satisfied a stable dependable combustion heater has been designed. The Testo 330-2 is a gas analyzer which measures the flue output and from that data calculates the efficiency of the burn, and can be connected to a computer as I have done to generate its own line diagram and spreadsheets.
'The test represented by the above diagram has been run in a warm heater, as is visible on the high start temperature. The draw of the chimney stack is therefore very strong and the fire is developing rapidly. The oxygen level (the green line) drops beneath what I do consider as the critical border of 6% O2. Below this border the course of the run can still be nice but chances are higher there will be a steep peak of carbonmonoxide (the purple line). Both the level of oxygen and the temperature of the exhaust gases (the blue line) have their influence on the efficiency (the red line).
'When oxygen isn't dropping any lower than 10% but on the other hand the end temperature isn't any higher than 80 degrees Celsius (176 Fahrenheit) the efficiency will be higher than it appears to be in the diagram above. Lower temperature of the exhaust gases means also less strong draft and that has its repercussions on combustion because of the lower gas velocity.
The test shown in the above diagram has been run in a warm heater, as can be deduced by the starting temperature (measured in the flue outlet, represented by the blue line). As mentioned earlier, a 'hot' (already up to working temperature) flue will have a correspondingly strong draw, and so in this run the fire developed quickly. During the run the oxygen level (green line) dropped below what I consider as the border between optimum and non optimum (6% O2). Below that figure runs the chance of higher (purple line) CO outputs (as insufficient oxygen is present). That did not happen in this case as can be seen, so whilst that risk is present it is clear that excellent burns can still be obtained. From all of the earlier discussion given on combustion it can be seen/understood that the oxygen levels, and the flue temperature are directly linked to the efficiency of the burn. The efficiency is shown by the red line.
When the oxygen content does not drop below 10% with a correspondingly low flue temperature of 80 degrees celsius (176 F) the efficiency will be higher than shown in the graph. (graph generated? or just this example). However lower exhaust gas temperatures means less draft and can have repercussions on combustion because of lower flow velocity through the system. (That kinda is contradictory? Yeah, I know the flow will slow, but high efficiency is claimed by these conditions, yet these conditions carry a 'warning', almost to the point of saying 'don't get these conditions'. I basically just copied it as written, just checking if some meaning got lost in the initial posting)
'It may be clear all conditions and settings are closely knitted together, it isn't possible to change a single aspect and leave all the others intact. A new situation will emerge which whether or not will react the same as the described experimental combustion unit. A new situation means starting completely over, building and testing in order to sort out the best option.'
These graphs that show the inter-relationship between the various parameters of the burn are a graphical way to understand the 'Important Point' made above, that it is doubtful that a departure from the descriptions given here will result in a more optimum heater. Of course it IS possible, but highly unlikely. The interactions within the heater are very complex, and any change has to be evaluated by actual measurement by testing similar to that shown here.
'Strangely enough having too much oxygen than required for complete combustion not only doesn't contribute TO the combustion, by definition, but also cools the fire. During development of what is presented in these pages naturally all permutations have occurred, including very bad runs during the trial and error tests, see the example below. It is only thru the Testo that we have arrived at a 'build by numbers' system presented here.'
this appeared in the earlier re-write, but has since been covered in depth just above. It is probably no longer needed?
That looks about it. Change, keep or modify as needed.
|
|
|
|
Post by peterberg on May 31, 2016 2:29:49 GMT -8
Pfew! This was quite difficult to keep track of all the changes. Please read through it all carefully whether this is what you expected to see. The first "Contact" reply has been arrived, an offer to do a Spanish translation!
|
|
|
|
Post by peterberg on May 31, 2016 10:06:06 GMT -8
My 'technical advisor' had some spare time and created the Spanish section this afternoon. The guy from Argentina, Pablo Kulbaba, translated the Introduction and I published it on the site. Looking very cool, three languages! Although the third one is just a beginning.
|
|
terry
Junior Member
 
Posts: 128 
|
Post by terry on May 31, 2016 12:37:25 GMT -8
Pfew! This was quite difficult to keep track of all the changes. Please read through it all carefully whether this is what you expected to see. The first "Contact" reply has been arrived, an offer to do a Spanish translation! yeah, I bet. I was getting lost myself tracking back and forth, it really does help to see it on your site rather than a forum editor! Looks like we salvaged it all, it seems to flow along nicely and all of the points in the different sections managed to get covered, and the dreaded triple french opening doors is not too obviously present. The only lingering thing, for me, is that it sounds very much/too much like me. Not an awful lot we can do about that, and I doubt it is that much of a problem that it needs addressing. The next page/chapter miiight need just a little bit, I think that was still done a bit tentatively, the ones after that I think should be fine (in the sense that any re-writes were being done more freely then). The only thing remaining to check (apart from any on the following page) is 'points left hanging' for lack of a better term. I will have to make notes, but as a quick example on the next page you said something like 'all heaters with a p channel are lit by small kindling just before the port, and when burning load the remainder' or words to that effect. That jars with something said later (pretty sure) that the preferred method is load the full load, start a small fire on top as far back as possible, let it consume the full load from top down. things like that. we need to ensure consistency not only within a page-which is what we have been concentrating on up till now-but also 'across' pages. hope that made sense. also we need a chapter/section on bell sizing and how to estimate that. We have just mentioned how much heat/unit of time these heaters give out, and as such how much area a steel drum needs to shed that heat or how we have to capture that heat in masonry. As these heaters are a system, those rules of thumb/guidelines need to be present. BUT, I think the finishing line is fast approaching, at least for what is currently there. |
|
|
|
Post by peterberg on Jun 1, 2016 4:42:20 GMT -8
The only lingering thing, for me, is that it sounds very much/too much like me. Not an awful lot we can do about that, and I doubt it is that much of a problem that it needs addressing. Translations to other languages are derived from the English version rather than from Dutch, so it is important to have the English version just right. When it sounds like you, so be it, I'm inclined to think it is a minor point. I mentioned you on the Introduction page as the editor/corrector and Pablo Kulbaba translated that line also, adding himself as the Spanish translator as well. This is how it ought to be, everybody who contributed significantly should be credited for it. The only thing remaining to check (apart from any on the following page) is 'points left hanging' for lack of a better term. I will have to make notes, but as a quick example on the next page you said something like 'all heaters with a p channel are lit by small kindling just before the port, and when burning load the remainder' or words to that effect. That jars with something said later (pretty sure) that the preferred method is load the full load, start a small fire on top as far back as possible, let it consume the full load from top down. The p-channel version is best lit in front of the port and loaded on top of that. The top-down-burn method works best for the floor channel variant. It is covered in the "Builds" chapter, please look into it whether or not this is sufficiently covered. also we need a chapter/section on bell sizing and how to estimate that. We have just mentioned how much heat/unit of time these heaters give out, and as such how much area a steel drum needs to shed that heat or how we have to capture that heat in masonry. As these heaters are a system, those rules of thumb/guidelines need to be present. This is definitely an item that need to be adressed, most likely somewhere in the "Bell" paragraph. I'll see into that coming weekend(ish). |
|
terry
Junior Member
 
Posts: 128 
|
Post by terry on Jun 1, 2016 14:05:23 GMT -8
well blow me down peter, you are right. 'In contrast with the normal implementation this version should be lit on top of the fuel pile at the back, known as "upside down firing". This method will deliver the best results.' I'd forgotten the rider of 'in contrast' in my hazy recollection, and in all likelihood I put it there in the first place! A perfect excuse to segue into another tiny discussion of how people learn, or what hinders that learning. As mentioned somewhere else, 'we' don't get it first time through, the more new or unusual, or even simply 'contrary to accepted knowledge' (and a smokeless fire that burns sideways with an exhaust temperature cooler than hot water is likely to be 'contrary wisdom' to most people), the more times through is needed before understanding. I am a bit unsure why you are so disinclined to include a general view, or an exploded view of these heaters. Made even more incomprehensible because of the sheer number of drawings used to show things throughout the site. (yes, I know I am violating the 'last time I will mention it', so at least I am aware I am being hypocritical). As far as I can remember, you did not want pictures to be included in your introductory 'bullet points' which is fine. Why not simply add a new 'bullet point' header, along the lines of 'General layout of the batch box rocket heater. This design is new to most people, so a general layout and exploded view is shown as an aid to understanding. (read more)' Or whatever the words might be. The same format has been followed, no picture is in the wrong place, and people can at least see this new exotic beast in a picture as opposed to merely words. I think you would concede that even if the picture did not really help, it most certainly does not harm? Or alternatively, even if the first hundred new readers did not need the picture to understand then no harm done, the one hundredth and first however..... I have often wondered how people in england would have pictured a kangaroo from the descriptions sent back home to them in the early days of white settlement here in australia. Or a platypus. What weird mental image picture would they have conjured up? That is re-inforced when you look at some of the earliest drawings/paintings done of kangaroos, they look like a cross between a rabbit and a rat, completely odd! Honestly, some of them are truly bizarre. AND this is from a painter (who's job is to paint what is in front of them) painting from real life! The whole notion of a kangaroo was so out of keeping with anything that had gone before that it even influenced their painting! (that actually fascinates me, hope it is interesting to others too) So yeah, trying to explain new things to people, especially when it conflicts with earlier thoughts on the matter, needs these sorts of aids. That REALLY is the last I'll speak of it, don't want to sound like a nagging fish wife. One thing I would like to add, for completeness or honesty. Forget exactly where it is, you will find it I am sure, where we talked about complete combustion..'all of the wood can be converted into heat' or very similar. I would follow that with an asterisk '...converted into heat'* ' * Whilst this is strictly true, a few points must be understood to put this into a real world context. These heaters will not be used in a laboratory with pure oxygen, they will be used at home. Even weather conditions will vary and influence the burn. Most importantly, all air dried wood will contain some moisture. This needs to be driven off before combustion of the wood can take place. To boil off water takes a tremendous amount of energy.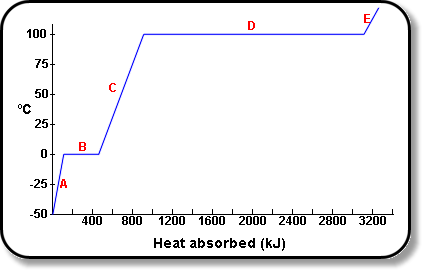
Point D shows where water (at 100 degrees) is converted to steam (at 100 degrees, the same temperature), and the huge amount of energy required to do so is seen, much much more than the energy required to take it from 0 degrees to 100 degrees (line C).
Unless we have a condensing boiler, that energy required to turn the water to steam is lost to the system, and into the atmosphere. So, in real world practice, these are some of the losses that do and will occur.
The *very*important*point* to be taken from this is, NEVER burn anything other than dry wood. Now you know why.' You may not want the diagram, and you may want to trim the wordiness, but I feel that little explanation is needed for completeness and accuracy. After all, 'our' own testo diagrams to do not show complete conversion of wood to heat! One last thing for you to consider as an addition somewhere. Again about learning, but a different aspect not yet covered. Often, a 'new field' of learning can be very overwhelming indeed. There is simply 'too much new data' to be able to absorb, be sorted out in the mind and from there be used. This is different from the conceptual grasping I have been banging on about. SOooo, I think a 'how to use' wrap up would be beneficial. You know, 'Now that you have come to the end of the description, how do you use this data?' So put yourself in their shoes and outline the steps YOU (personally) go through when designing a heater for a 'customer'. In other words, you would determine their needs (heat output, placement in the centre of the room, or in the corner, or against a wall), then you would mentally scan thru all the permutations given on these pages (shallow depth means sidewinder, yada yada). That then tells you WHICH dimension to choose from the table, whether it is single bell or double bell, yada yada. Think of it in terms of a flow diagram (unless the english term is foreign I am sure you know what that is) and it will be clear what I am talking about, and hopefully very clear how that will help a person actually apply all of this data given. Which I am sure is the whole intent of this website. Sure, it is easy for me to give you 'yet another assignment', but I think you would see the helpfulness of it? |
|
|
|
Post by peterberg on Jun 2, 2016 1:18:26 GMT -8
I am a bit unsure why you are so disinclined to include a general view, or an exploded view of these heaters. Made even more incomprehensible because of the sheer number of drawings used to show things throughout the site. (yes, I know I am violating the 'last time I will mention it', so at least I am aware I am being hypocritical). As far as I can remember, you did not want pictures to be included in your introductory 'bullet points' which is fine. Why not simply add a new 'bullet point' header, along the lines of 'General layout of the batch box rocket heater. This design is new to most people, so a general layout and exploded view is shown as an aid to understanding. (read more)' Don't worry, it is on my "to do" list, together with the dimensioning of a bell relative to the size of the core. The idea to add a new bullet point is good, I will use that. Don't expect it ot be done any time soon. One thing I would like to add, for completeness or honesty. Forget exactly where it is, you will find it I am sure, where we talked about complete combustion..'all of the wood can be converted into heat' or very similar. I would follow that with an asterisk '...converted into heat'* (snip) You may not want the diagram, and you may want to trim the wordiness, but I feel that little explanation is needed for completeness and accuracy. After all, 'our' own testo diagrams to do not show complete conversion of wood to heat! Good addition, but to me, it is not entirely clear what you mean, it looks like there's a flaw in the reasoning, to be honest. I think I am able to read a lot of this stuff in the English idioma but I fail to see the point. Would you care to read it over and clear it up a bit, just for me?  SOooo, I think a 'how to use' wrap up would be beneficial. You know, 'Now that you have come to the end of the description, how do you use this data?' So put yourself in their shoes and outline the steps YOU (personally) go through when designing a heater for a 'customer'. In other words, you would determine their needs (heat output, placement in the centre of the room, or in the corner, or against a wall), then you would mentally scan thru all the permutations given on these pages (shallow depth means sidewinder, yada yada). That then tells you WHICH dimension to choose from the table, whether it is single bell or double bell, yada yada. I think I get it, but what I should write about it is clear as mud to me. You see, for me such a process isn't one of just ticking the right boxes. What I do is giving it time, sleeping over it, brooding over it, parking it somewhere at the back of my mind for weeks or months. And then, most of the time, all of a sudden I see before my eyes what need to be done to solve the puzzle. The process is largely unconciously, waiting for inspiration so to speak. I'll place it on the list but don't expect much of it. |
|
terry
Junior Member
 
Posts: 128 
|
Post by terry on Jun 2, 2016 2:41:17 GMT -8
(re the adding the asterisk) 'Good addition, but to me, it is not entirely clear what you mean, it looks like there's a flaw in the reasoning, to be honest. I think I am able to read a lot of this stuff in the English idioma but I fail to see the point. Would you care to read it over and clear it up a bit, just for me?  ' haha, I get what you mean when you say I don't get what you mean! Had a quick glance, did not see any obvious changes, so not completely sure what 'care to read it over and clear it up means'. Unless you mean re-read what I wrote? Boy, this can get messy at times! Being almost 180 degrees apart time wise does not help either. I may have got it wrong, but for now will assume you were not sure why I wanted the clarification. We stated that apart from one percent ash, all of the wood can be converted to heat. Ie one kg of wood (that we put in the fire) we can extract 99% (weight) directly converted to heat. That is not strictly true. It will most likely, or at best, contain 15 % moisture, which will NOT be converted to heat, indeed is a direct sink of heat as it needs to be converted to steam/vapour. If we built a condensing boiler that can extract the latent heat of vaporization then yes, we can get that heat back. But that is an added complexity that we do not/have not done. Indeed, the very testo diagrams posted show that we do not have 99% efficiency. It was just a little bit of housekeeping for 'the sake of being honest', with a little moral ending (don't burn anything other than fully dried wood) I somehow think you are fully aware of all of that, so am left with the idea I have not understood your actual question or what the flaw is you are referring to. |
|
|
|
Post by peterberg on Jun 2, 2016 4:14:31 GMT -8
Point D shows where water (at 100 degrees) is converted to steam (at 100 degrees, the same temperature), and the huge amount of energy required to do so is seen, much much more than the energy required to take it from 0 degrees to 100 degrees (line C).
The above is not very clear, could you use other wording? As I understand it, it costs a certain amount of energy to take the water from 0 to 100 degrees. Then, to get it from 100 degrees to steam (the phase change) there's a huge amount of energy needed to achieve that. Maybe specifying the amounts would help?
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Jun 2, 2016 6:22:50 GMT -8
Hi Terry,
I'm working on the french translation and I came across the following sentence you wrote :
"Wood combusts completely with oxygen under specific circumstances (heat and ignition source), resulting in heat, CO2 and water."
The words in parenthesis are not right in my opinion because they imply that heat and an ignition source are enough to make a complete combustion, but maybe I misunderstood the sentence. What did you mean ?
thanks !
|
|




